
The pharmaceutical industry plays a crucial role in global healthcare, ensuring that life-saving medications and vaccines reach patients in a timely and safe manner. However, the logistics behind pharmaceutical supply chains are intricate and demanding, with unique challenges that must be overcome to maintain product integrity, compliance, and patient safety. In this article, we will explore some of the key challenges faced by the pharma logistics industry and examine the innovative solutions being deployed to address them.
Challenges in Pharma Logistics
1. Traceability and Visibility of Products
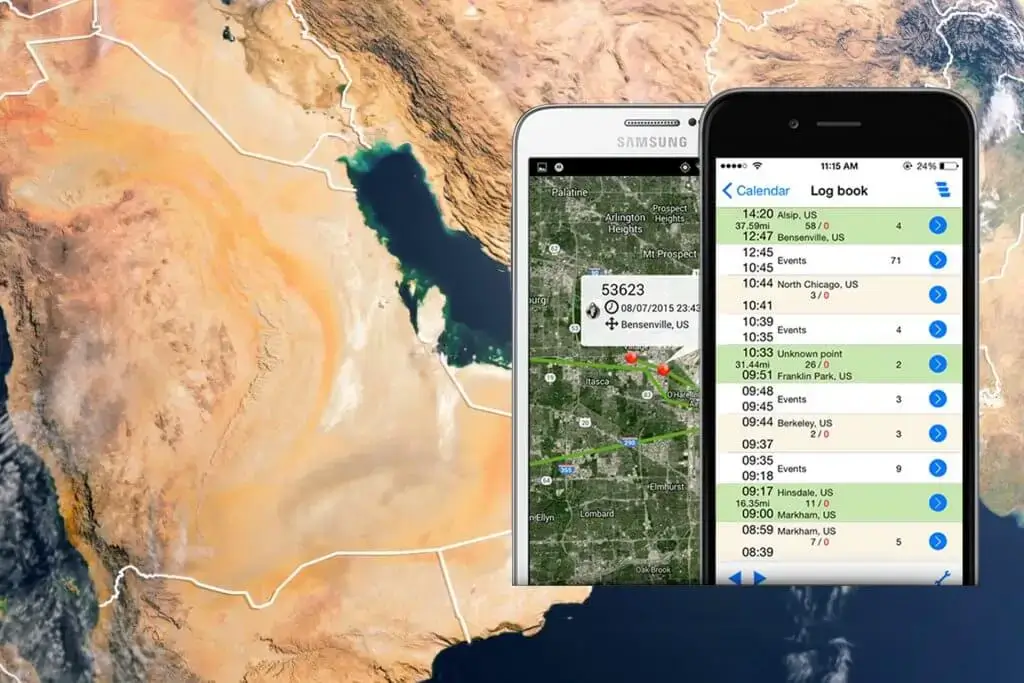
The ability to monitor the flow of goods across international boundaries is essential, especially when it comes to industries like the pharmaceutical. A game-changer in the area of product integrity is being able to pinpoint the exact location of your things. Every manufacturing company, but especially pharmaceutical companies, desires to maintain a tight eye on where its products are. This attention to detail includes not only ensuring that the items arrive at their destination but also preventing any mishaps or theft on the highway.
The demand for product visibility has increased in the current business environment as a result of the rise in third-party logistics, affiliate marketing, and the thriving e-commerce industry. Pharmaceutical producers in particular go through a rigorous procedure throughout the whole life of their products, from production to getting into the hands of the final user. Historically, businesses frequently only possessed knowledge of the dates when a product left their plant and arrived at its destination. Pharmaceutical firms cannot, however, afford to have such a narrow viewpoint in the era of strict rules and quality control.
Solution:

The GD100 arises as a ground-breaking response to the increased need for real time monitoring for product visibility, particularly in sectors like medicines. The difficulties of tracing items across international borders can be overcome with the help of this one-time use GPS monitoring gadget. It not only tackles security issues but also simplifies the complicated realm of reverse logistics. Particularly pharmaceutical businesses have long wished to monitor their goods at every stage of their journey, from manufacture to the consumer’s hands.
GD100 has a built-in light sensor and real-time GPS tracking, so it can identify instances of package tampering or unauthorized opening. When such a situation arises, the gadget immediately sends a notice with the Google map position, time, and date stamped. This makes sure that any possible interruptions or security breaches are swiftly found and dealt with.
Additionally, ROUTEWATCH offers real-time information on the device’s position, time, date, weather, and even the distance and journey time. With the help of this invention, pharmaceutical businesses can now retain the safety and authenticity of their medicines while simultaneously meeting strict requirements and ensuring smooth logistics. The GD100 and routewatch offer a comprehensive solution that protects reputation and ensures customer safety in today’s age of increased scrutiny and quality control.
2. Temperature Sensitivity:

Several items in the supply chain for medical and pharmaceutical products are susceptible to small environmental changes, such as temperature fluctuations and inappropriate handling. Any stage of the process, including the procurement of raw materials, transportation, and the administration of medications to patients, might include improper handling.
Failures in temperature-controlled logistics alone cost the pharmaceutical sector over $35 billion annually. Even a small temperature difference from the usual might make a cargo unusable and result in an expensive write-off. Care must also be used when handling pharmaceuticals and medical supplies. Medical equipment might contain delicate moving components and some pharmaceuticals may be packaged in glass bottles. In the industry, rough treatment is a surefire way to write anything off.
Organizations must closely monitor for and prevent supply chain mistakes since they can alter product chemistry, harm packaging, or have other negative effects on product safety. Manual monitoring options take a lot of work and paperwork.
Solution:

Organizations used temperature-controlled containers and monitoring systems to address these issues. In addition to providing real-time data for tracking and correcting any deviations, these cutting-edge solutions are essential for ensuring that the proper temperature range is maintained during transit. For instance, Peli BioThermal is a prominent example, providing the worldwide life sciences sector with a wide range of temperature-controlled, thermally protected packaging and service solutions.
The CoolGuardTM Advance, one of their notable inventions, offers a substantial advancement in temperature-controlled transportation. It converts each shipping container into a fortress of temperature stability using a combination of phase change materials (PCM) and vacuum insulated panels (VIP), extending the protection window to an astonishing 72 to 120+ hours. Pharmaceutical and biotechnological businesses, which place a premium on product integrity, need this prolonged duration more than any other.
The Credo CubeTM, which redefines temperature-controlled shipping with a comprehensive strategy, is another outstanding solution. This passive, reusable design not only promotes environmental stewardship but also protects priceless medicinal resources. It guarantees that even the most fragile goods reaches in perfect condition by maintaining ideal temperatures for up to five days while lowering the carbon impact and total transportation expenses. The pharmaceutical industry’s dedication to maintaining product safety and sustainability in the face of a complicated and developing supply chain is exemplified by these cutting-edge technologies.
3. Real-time data collection

One of the biggest obstacles in the pharmaceutical supply chain is the difficulty of keeping product integrity while in transit. Anomalies, particularly those connected to temperature excursions, might take longer to discover and manage when there is a lack of real-time data on your items. This lack of understanding might make it more difficult to identify the main reasons for delays or damage, leading to an expensive blind spot. Additionally, the lack of real-time data makes it extremely difficult to manage resources effectively and guarantee that shipments are handled with the highest care, since you are left in the dark regarding the expected time of arrival (ETA).
Solution:

The pharmaceutical sector is utilizing cutting-edge cold chain temperature control systems to address these urgent issues. The pioneer in creating cutting-edge environmental monitoring systems suited to the pharmaceutical, life science, biotech, and healthcare industries is ELPRO, a Swiss-based global leader in the industry.
A ground-breaking solution that can reduce losses and improve the integrity of pharmaceutical shipments is one of their main products, the LIBERO CL. This adaptable PDF Logger with several levels and uses is furnished with inbuilt temperature sensors in addition to USB and Bluetooth® connections. The internal temperature sensor gives remarkable precision and a wide working range, ranging from -30 °C to +70 °C, supported by a 100% sensor calibration.
The smooth accessibility and data retrieval of LIBERO CL makes it stand out. Users have the option of downloading the PDF report through the easy USB interface, guaranteeing usability and system compatibility. Users can also simply download the PDF report to their smart devices via the Bluetooth® interface. This cutting-edge technology is revolutionary because it provides supply chain managers with real-time access to crucial data, allowing them to monitor and respond to temperature and environmental conditions while shipments are in route.
Additionally, ELPRO adds advanced monitoring systems and smart database solutions to their product offerings, creating a holistic package that complies with Good Practice (GxP) requirements. This level of assistance covers the crucial facets of data management and compliance in addition to the product itself.
Pharmaceutical firms may reinforce their supply chains with real-time data and reliable temperature control by deploying next-generation systems like LIBERO CL. This improves their capacity to see abnormalities quickly and deal with them, as well as how resources are allocated, how shipments are handled effectively, and finally how the integrity of the product is protected.
4. Regulatory Compliance:

In the healthcare industry, mistakes are not allowed. Strict government and business standards set forth requirements for processes, quality standards, and documentation. Although necessary for ensuring public safety, these expectations create problems in the medical supply chain. Continuous visibility may be difficult, but if it’s the only way to make sure a medicine is still functioning when it’s needed, it’s worth the hassle.
Regulations that are always changing as well as requirements for international commerce are major compliance factors that the pharmaceutical business must take into account. Regulations may also include standards for recordkeeping, packing, handling techniques, and labeling, in addition to environmental factors like temperature and humidity. For instance, calibration requirements set by the National Institute of Standards and Technology (NIST) must be met by temperature and humidity monitoring sensors.
Solution:

Strict government and commercial standards are the law when it comes to procedures, quality standards, and documentation in the healthcare sector because the margin for mistake is practically nonexistent.
Pharmaceutical logistics businesses must invest significantly in quality management systems, standard operating procedures, and thorough staff training programs in order to negotiate the complicated terrain of changing legislation and the requirements of global trade. These serve as the cornerstones for guaranteeing regulatory compliance and upholding the highest levels of quality and safety.
Additionally, since rules are always changing, strong cooperation with regulatory bodies is essential. This requires keeping a pulse on the constantly changing laws and regulations. All elements of cold chain logistics, including packing supplies, tools, and delivery vehicles, must adhere to the necessary requirements. To ensure transparency and compliance preparation for any audit or inspection, documentation should be carefully stored and easily available.
However, the pharmaceutical business should adopt best practices for cold chain logistics in addition to compliance. This entails thorough employee training on how to handle and transport pharmaceutical items safely, the development of effective risk management plans, and frequent audits to make sure every link in the cold chain is operating perfectly. Contact us now for a friendly initial consultation.
5. Global Supply Chain Complexity:

Pharmaceutical businesses operate in a worldwide setting where the game’s rules might vary from one country to the next. Pharmaceutical import, export, and distribution are governed by a unique set of laws in each nation, which are frequently mandated to protect public health and uphold high standards of quality. These rules cover a wide range of topics, including product labeling and registration requirements, temperature control, and security procedures.
The inherent complexity of these international regulations can provide serious logistical difficulties for the pharmaceutical industry. Given that many medicines are time-sensitive, it becomes more challenging to make sure that patients receive them in a timely and effective manner. In addition to having a severe financial impact on pharmaceutical companies, supply chain delays or errors can seriously injure patients.
Solution:

A major development in the pharmaceutical sector is the formation of cooperative relationships between pharmaceutical firms and logistics service providers in response to these diverse issues. These collaborations are demonstrating to be a tactical response to the complex dynamics of the pharmaceutical supply chain.
A multidimensional strategy for optimizing pharmaceutical supply chains is collaboration. They encourage the sharing of knowledge, assets, and best practices between logistics service providers and pharmaceutical businesses. These partnerships stress a mutually beneficial cooperation where both parties actively contribute to enhancing supply chain operations rather than the conventional vendor-client relationship.
The capacity to adjust to local market conditions is one of the key benefits of such collaborations. Pharmaceutical firms learn about the particular requirements and difficulties of each market they serve when they collaborate closely with logistics service providers. This understanding enables the creation of customized solutions that not only satisfy legal standards but also take into account the unique characteristics of each individual location. The timely distribution of pharmaceutical supplies and the reduction of interruptions may both be achieved with the use of such adaptive solutions.
Conclusion
The pharmaceutical sector is resolute in its commitment to patient health, but the logistical process is fraught with difficulties. Key obstacles include the requirement for real-time visibility, tighter rules, and changing global needs.
The sector makes investments in strict quality control procedures, personnel development, and collaborations with logistics service providers to address these difficulties. These collaborations make it possible to share resources and experience, ensuring that operations are tailored to the needs of regional markets.
Cold chain temperature control innovations, including ELPRO’s LIBERO CL, provide internal temperature sensors and real-time data access. These systems enable quick decision-making and effective shipping management while preserving product quality.
For the smooth delivery of life-saving pharmaceuticals, pharmaceutical logistics is embracing innovation, teamwork, and a patient-first perspective. These techniques hold the key to a future where accuracy and patient safety are paramount in a sector where mistakes are not accepted.
If you’re interested in finding out more about the Solutions for pharma logistics problems contact us at coldchainpacking.com today!